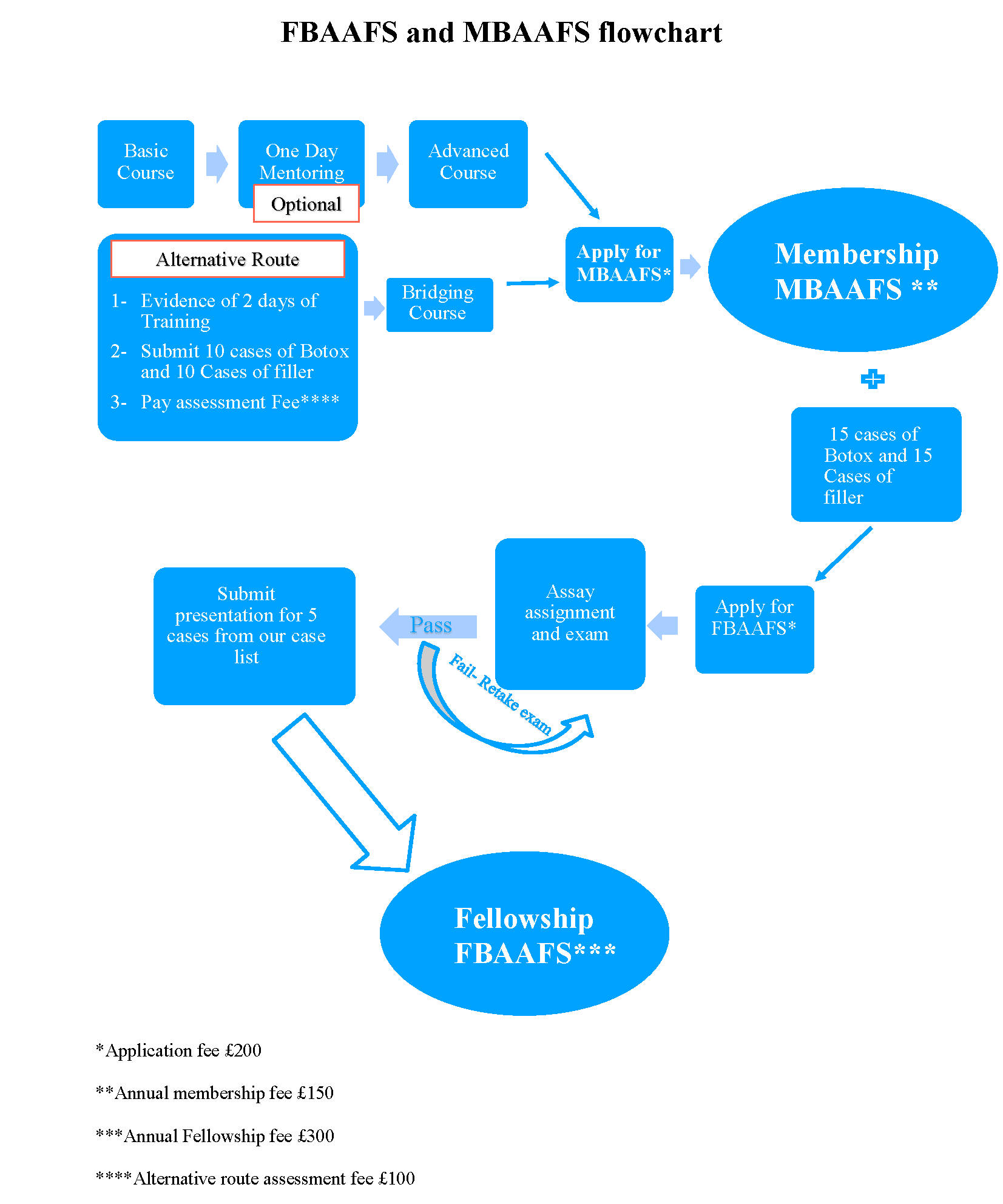
Route to Fellowship of the British Academy of Aesthetic Facial Surgery (FBAAFS)
- Upon successful entry to BAAFS membership and completion of 30 advanced cases of botulinum toxin and filler treatments, members can apply to become a recognised fellow of the BAAFS.
- At This stage members can arrange a day of mentoring to augment their skills and make up any additional treatments required for FBAAFS.
- Each application is assessed individually by one of our expert caseworkers. If the application is successful, members will be asked submit a 3500-4000 word essay in a subject from within one of the following areas:
1: History of Aesthetic medicine, Ethics and law
2: Psychological considerations in aesthetic medicine
3: Principles of Treatment in Aesthetic Medicine
4: Anatomy and Dermatological considerations
5: Neurotoxins in aesthetic medicine
6: Practical use of Neurotoxins in aesthetic medicine
7: Dermal fillers in aesthetic medicine
8: Practical use of dermal fillers in aesthetic medicine
- Upon successful completion of the essay. Applicants will be asked to prepare a presentation of 5 cases from the following list of procedures:
- Upper face botulinum toxin
- Midface botulinum toxin
- Lower face botulinum toxin
- Nasolabial fold filler
- Marionette lines filler
- Upper cheek filler
- Lower cheek filler
- Pre-jowl sulcus treatment
- Chin filler
- Lip filler
- Nose filler
- Tear trough filler
- Neck treatment
- Jawline filler
- Use of Cannula
- Case presentations should be submitted in PowerPoint format and must include: history, examination, findings, treatment plan, preoperative and postoperative photographs.
- Following submission of the powerpoint presentation. A viva assessment will be held to assess the applicant’s depth of knowledge on cases presented.
- After admittance to Fellowship, in order to maintain their FBAAFS status, Fellows are required to submit an annual evidence of:
- 50 hours of CPD (with minimum 10 verifiable hours on Aesthetics), or
- 10 verifiable hours of CPD on Aesthetics together with Certificate of good standing from relevant regulatory body.